Recency Testing at IAS 2019
by Dr. George Rutherford
University of California, San Francisco (UCSF)
Recency testing using the limiting antigen (LAg) avidity assay was one of several new breakthroughs in HIV control discussed at the 10th International AIDS Society Conference on HIV Science, which was held in Mexico City from Sunday, July 21, 2019 to Wednesday, July 24, 2019. The conference kicked off with a satellite session sponsored by TRACE partners UCSF and ICAP.
The satellite session included opening remarks by Ambassador Deborah Birx where she shared her vision for expanding recency testing in routine settings to all PEPFAR countries. She emphasized the urgency to understanding the epidemic and responding in real-time in order to achieve our goals of epidemic control. Check out the video of her opening remarks on the TRACE eLearning hub here. The session continued with presentations sharing experiences from Rwanda, Ethiopia, Malawi, and Vietnam, all countries currently implementing recency testing. The last hour of the session was a panel discussion on using recency data to benefit PLHIV, ethical considerations, and program implications.
The session was moderated by Andrea Kim, one of the TRACE co-leads from CDC. The panel was comprised of Amalia Girón from Universidad de la Valle, Guatemala where they have been doing recency testing and returning results for over a year; Rachel Baggaley, who is working on WHO guidance regarding the use of and ethical implications of recency testing; Gregorio Millett from AmfAR, who represented the views of the community; Wame Mosime from ITPC, who brought perspectives from grass roots community organizations; and Jerome Singh, an ethicist from University of KwaZulu-Natal who brought the perspective of ethical and legal implications. The panel discussed the use cases of recency results at the individual, surveillance, and program levels and additional scientific evidence that is needed to understand the benefits/risks of returning individual results. Finally, the panel discussed how to engage the PLHIV in rolling out recency testing. The panel discussion will soon be posted on the TRACE eLearning hub as well.
In addition to the panel discussion, there were multiple abstracts, symposia, and oral presentations which dealt with incident infection, partner notification, investigation of HIV clusters, and genotypic linkage of newly diagnosed cases. There was a relatively modest number that focused specifically on point-of-care testing for recent HIV infection using LAg avidity testing.
Point-of-care LAg avidity testing was featured in two presentations, one a poster presentation from the MeSH Consortium at the London School of Hygiene and Tropical Medicine[1] and another oral presentation from the Vietnam AIDS Authority Council (VAAC)[2] in an oral abstract session entitled “Interrupting transmission using new tools.”
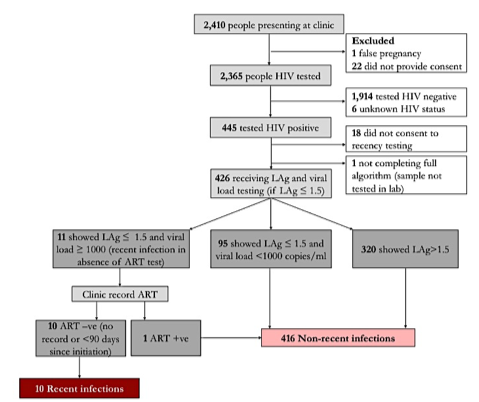
In the first of these, Risher and colleagues reported on data from a study conducted among pregnant women attending antenatal clinics (ANC) in western Kenya.1 The investigators compared incidence estimates in adolescent girls and women ≥13 years old attending one of 14 ANC derived from LAg avidity testing with incidence estimates from women who could be matched to serial seroprevalence surveys from the Health and Demographic Surveillance Site (HDSS) that covers the Gem district. HDSS data are often viewed as the gold standard for population-based estimates. Seropositive ANC patients were tested recent infection using the Maxim HIV-1 LAg Avidity EIA® and the recent infection testing algorithm (RITA) method (both viral load testing and matching clinic records for antiretroviral therapy); for DSS participants, incidence was based on known seroconversions. Using LAg avidity testing and RITA, of 426 women with newly diagnosed HIV infection, 10 (2.3%) were found to have recent infection (Figure 1). This equated to an incidence in this specific population of 1.1 per 100 person years. Among ANC patients who participated in the HDSS, there were 25 seroconversions in 1,877 person years of follow up and an estimated incidence of 1.3 per 100 person years. Thus, the results obtained from LAg avidity testing and directly observed seroconversions in the same population were very similar. Additionally, the investigators reported that women in their first trimester of pregnancy has 9.6 times the adjusted odds of testing positive for recent infection compared to women in their second and third trimesters. The investigators concluded that (1) the acceptance rate for recency testing was high, (2) HIV incidence estimates using LAg and RITA were similar to those obtained from linked seroconversions at the local HDSS site, and (3) LAg testing with RITA is a promising avenue for ANC surveillance, where the population at risk can be clearly defined.
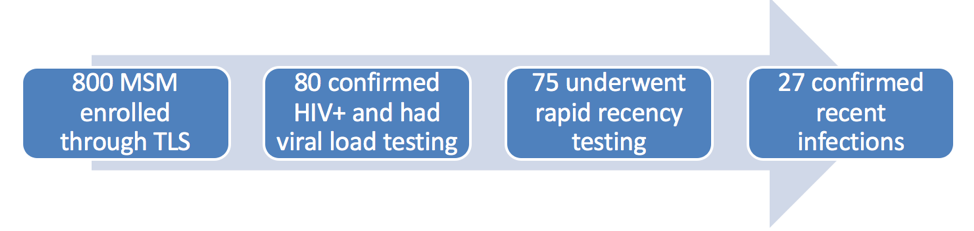
In the Vietnamese study, 2 Vu and colleagues reported results from a cohort study of men who have sex with men (MSM) living in Hanoi and recruited through time-location sampling. Inclusion criteria were being a Vietnamese national, male at birth, ≥16 years old, having lived in Hanoi ≥3 months, and having had oral or anal sex with another man or transgendered women in the past 12 months. Baseline specimens were tested for HIV infection using three third- and fourth-generation tests serially, then specimens testing positive for HIV on both were tested for viral load. Then those with viral load <1,000 copies/mL were tested for recent infection using the Sedia Asanté HIV-1 Rapid Recency® assay. Note, that this is the reverse of the usual algorithm where viral load testing is done only for those with positive recency assays. They derived incidence estimates using SACEMA R inctools package (http://www.incidence-estimation.org/) using a mean duration of recent infection of 161 days and a false positive rate of 0%. Of the 800 men in the cohort, 80 (10.9% when weighted for study design) tested positive for HIV infection, and 75 continued with further testing. Of these, 27 (36%) had confirmed recent infection (Figure 2). This corresponded to an annual incidence of 5.8 per 100 person years (0.8-10.6 per 100 person years). In multivariate analysis, men who had been recently infected had higher adjusted odds than men who had been infected more than one year previously to be low income, to not have been exposed to chemsex, and to not have syphilis. The prevalence and incidence found in this study are substantially higher than results based on the Asian Epidemic Model (4.8% and 0.36 per 100 person years). The investigators will extend recency screening to the other two arms of this cohort (MSM recruited by respondent-driven sampling and MSM recruited by Internet-based respondent-driven sampling), and Vietnam will be scaling up recency testing more broadly in the coming year.
In addition to these two studies that utilized point-of-care LAg avidity assays, three other studies used conventional LAg avidity testing to estimated incidence. These studies came from cohorts of MSM and transgender women in Tijuana, Mexico,[3] adolescent girls and young women in five southern African countries,[4] and injection drug users in Athens, Greece.[5] Both the Mexican and the Greek studies used recency testing with RITA to identify recently infected cohort members and then to use them to construct network analyses.
In the Mexican study, Proyecto Enclaces, investigators recruited a new cohort of MSM and transgender women using venue-based sampling, partners of infected cohort members identified through contact tracing and respondent-driven sampling.3 Inclusion criteria were age ≥18 years, anal sex with a man or a transgender woman in the past 4 months, and either a self-reported HIV infection or a laboratory-confirmed infection. In addition to testing participants using the LAg avidity assay, samples were also used to generate pol-1 sequences by PCR and Sanger sequencing and then constructed a network map using TRAnsmission Cluster Engine (TRACE), a tool for large-scale molecular epidemiology. Of the 233 participants enrolled, 195 were tested for recent infection. Of these, 22 (11%) had positive LAg assays. Participants who had lived in Tijuana longer, those who had used cocaine in the past month and had ever experienced sexual abuse had higher adjusted odds of recent infection compared to older infection. Overall, 9 (60%) of 15 participants who had both LAg and pol-1 sequences clustered compared to 40 (31%) of 130 with more long-standing infection. The investigators concluded that because the recent infections were not linked to each other, there was most likely steady, ongoing transmission of HIV in this community in Tijuana.
In the Greek study,5 (9.8%) of highly connected core cohort members (k score=4 or 5) were recently infected compared to 3 (2.0%) of 147 participants with fewer connection (k score ≤3) (p=0.038).5 These investigators concluded that being in a more connected part of the risk network in this extensive outbreak was associated with recent infection as the outbreak slowed and transmission waned and suggested the utility of contact tracing from those with new infections to control outbreaks at least in the early stages.
The other study that utilized laboratory-based LAg avidity testing was from the Population-based HIV Impact Assessment (PHIA) program.4 This paper combined data on HIV-infected 15-to-24-year-old adolescent girls and young women (AGYW) and their cohabiting male partners from five southern African countries where PHIA surveys had been conducted: Eswatini, Lesotho, Malawi, Zambia, and Zimbabwe. Its focus was primarily on characterizing male partners of AGYW with prevalent infection. Recent infection was defined as LAg avidity assay positive, viral load >1,000 copies/mL, and no detectable antiretrovirals. HIV prevalence among both AGYW and their cohabiting male partners was 6%, and HIV incidence in AGYW ranged from 0 per 100 person years among those with 15-to-24-year-old male partners to 3.0 per 100 person years among those with 35-to-44-year-old male partners. The odds of recent AGYW infection increased 8% with each additional year of partner age difference (OR=1.08, 95%CI 1.05-1.11) and was associated with increasing viremia among older male partners but not age per se. The investigators concluded that improving ART coverage among key male age groups (25-44 years old) may advance HIV prevention among AGYW.
What are the take-home lessons? First, recency testing is largely acceptable to individuals seeking routine care and participating in studies. Secondly, in the two studies in which point-of-care testing was used, the test performed well. Third, and this was brought out in the question-and-answer session following the oral abstract session, incidence is only meaningful when there is a defined population from which the sample has been drawn. So, estimates of incidence from clinic samples, unless those samples are representative of a population (like all pregnant women in a specific area), should be taken with a grain of salt. In studies where a representative baseline sample is used, as in the Greek, Mexican, and 5-county PHIA study, incidence calculations are meaningful. Because of the likely representativeness of the Kenyan and Vietnamese studies, these calculations are also likely meaningful, especially in Kenya where they were compared against a known standard. Finally, examining cohorts for differences between those recently infection and those infected in the past is similarly tricky. People with long-term infection had recent infection a few years before, so the best way to interpret these data are as secular trends. Finally, recent infection can be used to construct network maps and improve our understanding of transmission dynamics. As more data come in from incident infection surveillance systems and index partner testing programs, the utility of recency testing for disease control purposes will become clearer.
1. Risher K, Ambia J, Calvert C, et al. Incidence in pregnant women attending antenatal clinics in western Kenya [MOPEC371]. 10th International AIDS Society Conference on HIV Science, Mexico City, Mexico, July 22, 2019.
2. Vu D, Le G, An L, et al. High burden of recent and prevalent infection among men who have sex with men (MSM) in Hanoi, Vietnam [WEAC0103]. 10th International AIDS Society Conference on HIV Science, Mexico City, Mexico, July 24, 2019.
3. Skaathun B, Pines HA, Patterson TL, et al. HIV incidence among men who have sex with men (MSM) and transgender women (TW) in Tijuana, Mexico [WEAC0106]. 10th International AIDS Society Conference on HIV Science, Mexico City, Mexico, July 24, 2019.
4. Ayton S, Schwitters A, Mantell J, et al. Male partner age and HIV infection among young women cohabiting partnerships in five countries in southern Africa [MOPEC344]. 10th International AIDS Society Conference on HIV Science, Mexico City, Mexico, July 24, 2019.
5. Friedman SR, Williams LD, Paraskevis D, et al. A highly-connected risk network position was associated with recent HIV infection approximately three years after the large HIV outbreak began among people who inject drugs (PWID) in Athens. {MOPEC334]. 10th International AIDS Society Conference on HIV Science, Mexico City, Mexico, July 24, 2019.
