KEY CONSIDERATIONS
Recency Assays and Algorithms
- Tests for recent infection (TRIs) can help classify persons newly diagnosed with HIV-1 infection as either recent or long-term based on maturation of antibody avidity (i.e., antibody binding strength) at the time of testing.
- Results of laboratory validation and field evaluations of rapid tests for recent infection (RTRI) demonstrate RTRI is comparable to LAg-Avidity EIA in detecting recent infections 1,2.
- RTRI should be performed by well-trained and certified staff and can be conducted at HIV testing location, in a health facility, or in a laboratory.
- Viral load testing is strongly recommended among those who test recent on TRI as part of recent infection testing algorithm (RITA). Viral load results <1000 copies/mL can be used to help reclassify individuals previously diagnosed and on ART and elite controllers as RITA-long- term.
Testing Clients for Recency
- Results of TRIs (whether recent or not) are only for surveillance use and do not alter HIV diagnosis status or care provided to newly HIV diagnosed persons.
- TRIs should be offered as an additional test to clients who are confirmed as newly diagnosed with HIV-1 based on the national HIV testing algorithm. HIV retesting among persons on ART may be a common practice, but may go unrecognized at the time of testing.
- TRIs should be offered to adults, generally ≥ 15 years age or age of consent for HIV diagnostic testing. Although there is no age specification for use of TRIs, persons <15 year of age are most likely to have been perinatally infected with HIV and previously diagnosed and on ART.
Recent HIV Infection Surveillance
- TRIs enable the establishment of HIV-1 recent infection surveillance. Recent infection surveillance can be used with other surveillance and program data sources to provide signals of ongoing HIV transmission to inform public health programming and response.
- At the population-level, routine analysis of recent infection surveillance data can help identify geographic areas and sub-populations associated with HIV recent infections and inform HIV prevention and care programming.
- World Health Organization has included HIV recent/long-term infection status as a reportable event on its model case report form (Annex 3.4.7, Consolidated guidelines on person-centered HIV patient monitoring and case surveillance, 2017). Countries are encouraged to capture HIV recent/long-term infection status in national HIV case reporting.
A test for recent infection (TRI) measures maturation of HIV antibody avidity (i.e., antibody binding strength). Avidity increases after seroconversion, as part of the immune response, and the timing of this maturation varies among individuals. A rapid test for recent infection (RTRI) can help classify HIV-1 infections as recent or long-term using a simple lateral flow format. If antibody avidity has matured beyond a designated threshold by the time of testing, the RTRI classifies the sample as ‘long-term’. Otherwise, the result is ‘RTRI-recent’. Because long-term viral suppression (antiretroviral therapy [ART] or elite controllers) tends to lower antibody avidity, TRIs are not recommended to be used alone to classify HIV infections.
A RITA is a combination of laboratory tests used to classify an HIV infection as recent or long-term. A RITA helps A RITA is a combination of laboratory tests used to classify an HIV infection as recent or long- term. A RITA helps to reduce false recent classification when individuals are on ART and virally suppressed or are elite controllers. If a client tests recent on the TRI, viral load (VL) testing should be conducted to improve accuracy of classification. Those who test recent on the TRI and have aviral load ≥1,000 copies/mL should be classified as RITA-recent (Figure).
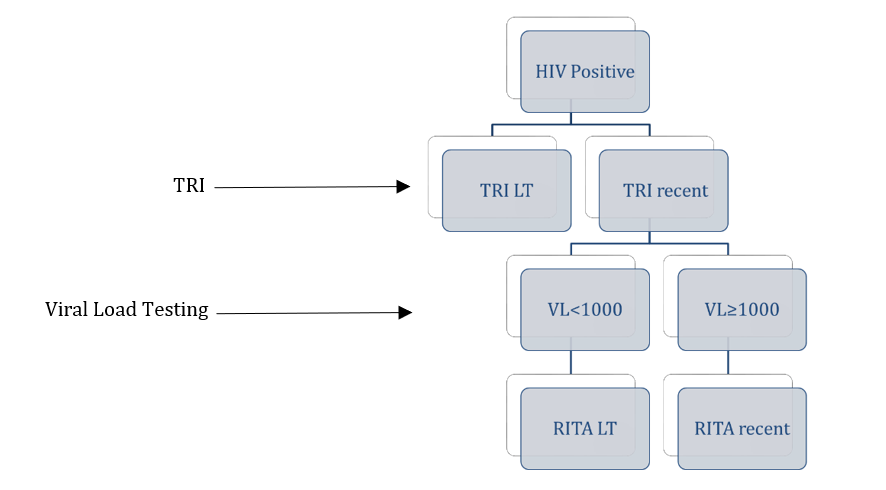
A proportion of persons on ART will test TRI-recent due to declining antibody avidity when the HIV viral load is A proportion of persons on ART will test TRI-recent due to declining antibody avidity when the HIV viral load is suppressed for a period of time. Similarly, some elite controllers may test TRI- recent because the low viral load does not support the development of strong antibody avidity. For these reasons, RITA is strongly recommended for all recent infection surveillance to improve the accuracy of RTRI classification.
Currently RTRI technology has been commercialized by two manufacturers as 1) Asante HIV-1 Rapid Recency Assay (Sedia Biosciences, Portland, OR, USA) and 2) Maxim Swift Recent Infection Assay (Maxim Biomedical Incorporated, Rockville, MD, USA). Asante is available as 100 tests or 20 tests per kit, while Maxim Swift RIA is available as 20 tests per kit. Both test formats have undergone extensive in-house evaluation and validation at CDC and independently by other laboratories. In all settings, the tests performed well with acceptable performances for recent infection detection, compared to LAg-Avidity EIA.
The International Laboratory Branch (ILB) within CDC is working with additional manufacturers to transfer this technology successfully. When RTRI kits from new manufacturers are validated and approved, additional notification will be shared.
Both RTRI and LAg-Avidity EIA use the same gp41 multi-subtype protein and are based on the same principle of using limiting antigen to distinguish recent from long-term infections. However, the LAg-EIA is a laboratory-based assay requiring specialized equipment, while RTRI is a rapid test in lateral flow format and can be performed at HIV testing location, in a health facility, or in a laboratory. The performance of RTRIs in distinguishing recent from long-term infection are similar to the LAg assay at normalized optical density (ODn) cutoff of 2.0 corresponding to the mean duration of recent infection (MDRI) of 6 months for the Asante RTRI), or LAg ODn cutoff of 1.5 corresponding to an MDRI of 4 months for the Maxim Swift. However, visual reading and interpretation of RTRI can be subjective and result in inter-operator variability. Both training and certification of persons conducting TRIs and continuous quality improvement and monitoring are strongly recommended during introduction and implementation of recent infection surveillance.
Yes. In addition to VL testing as a part of RITA, recency classification can also be improved by using clinical history to identify and exclude from TRI testing persons presenting to HTS with advanced HIV disease, low CD4+ T cell count, prior HIV diagnosis and prior/long-term ARV use. However, HIV diagnosis and subsequent ARV initiation with or without suppressed VL within the last 4 weeks of RTRI testing, should not affect the TRI result.
No. The RTRI was developed for the detection of recent HIV-1 infection. The long-term line of the test includes only HIV-1 specific antigen. Therefore, the RTRI cannot be used to detect recent infections among persons diagnosed with HIV-2. If used in geographical areas where HIV-2 is prevalent (or if HIV-2 infection is suspected), it is important to perform type-specific diagnosis using Geenius or similar test before final recent infection classification can be made.
Yes. The mean duration of recent infection is about 6 months, but there is a distribution around the mean, which can vary from ≤3 months to >9 months across individuals due to the variation in immune response. Based on this distribution, although we expect most persons who test RTRI- recent to have acquired HIV approximately within the last 12 months, there may be some who have had HIV for more than 12 months. Prior ART (particular for longer duration) or weak antibody development among elite controllers can increase the likelihood of testing false-RTRI-recent. The addition of viral load testing in a RITA can help identify and reclassify these persons. Additionally, clinical history can be used to identify and exclude from RTRI testing persons presenting to HTS with prior HIV diagnosis
Yes. Individuals transition from recent to long-term status at varying time periods after infection (≤3 to >9 months). This means that individuals may reach the antibody avidity threshold, but are still within 12 months of infection and will test as RTRI-LT.
No. The RTRI does not detect acute HIV infection. Persons who have acute HIV infection do not have HIV antibodies and will test HIV-negative by a national HIV testing algorithm that uses HIV antibody tests. Thus, in countries where the national HIV testing algorithm is based on antibody detections, persons with acute infection will not be detected and will not be offered recency testing or included in recent infection surveillance. In countries using both Ag/Ab combo tests (e.g. Determine Ag/Ab Combo), only persons with reactive antibody-positive results should be offered recency testing.
No. Unlike LAg-Avidity EIA, RTRI should not be performed using DBS. The RTRI should be performed using whole blood, plasma or serum specimens.
Although this may happen, it should be rare. In countries where regulation or policy permits, programs may consider repeating the national algorithm to confirm HIV-positive status (if retesting for verification was not done). In general, RTRI should be repeated and the results recorded. Irrespective of RTRI results, the national HIV testing algorithm is always the final HIV result. It is important to review inconclusive results in the context of total number of HIV-positives tested. From the review of >60,000 HIV-positive samples tested by Asante in multiple countries, almost 99.8% were classified either as long-term or recent with only 0.2% with inconclusive results.
Any visible line, even if weak, should be interpreted as present. The long-term line measures the evolving antibody avidity and a person could truly be at the transition point between recent and long-term infection. In this case, the test may show a faint long-term line which may be detected differently by different testers.
Yes. PrEP may slow maturation of antibodies, which can delay identification as HIV-positive on routine diagnostic tests and result in infection classified as recent for a longer time than the mean duration of recent infection (about 6 months). These events (breakthrough infection while on PrEP) are likely to be rare. Persons using PrEP who are newly diagnosed with HIV, are eligible for recency testing and inclusion in recent infection surveillance
Recent surveillance data can be used to characterize new HIV diagnoses in a population by recent infection status and other characteristics of interest routinely captured such as demographics or risk information. Additionally, these data can be used to estimate the proportion recently infected among a proxy population of persons at-risk (those who test HIV-negative within HTS plus those who are classified as recently infected). These analyses can help monitor for trends in the overall population or among specific subpopulations. Additionally, geospatial analysis of new HIV diagnoses by recency status can help to detect potential hotspots where there is ongoing transmission and need for further investigation to identify gaps and address them through program strengthening and response. Many of these analyses can be built into national recent infection surveillance dashboards to facilitate timely and routine data use by Ministries of Health and key stakeholders.
Many factors need to be carefully considered in use of routine recent infection surveillance to estimate population HIV incidence, including recency assay-specific performance characteristics, timing and coverage of HIV testing among persons at-risk within the population, and coverage and quality of recency testing among those newly diagnosed with HIV. Approaches that adequately measure or adjust for these factors may make it possible in the future to use routine recent infection surveillance to estimate HIV incidence.
No. TRIs are used for surveillance and not for HIV diagnosis or individual-level clinical management or care. WHO does not currently have a pathway for prequalification (PQ) assessment of any tests for recent infection surveillance or incidence estimation, including the RTRI and LAg- Avidity EIA assay.
Possibly. Some country regulating bodies may require registration of the RTRI prior to routine use, and registration requirements may differ based on the intended use (e.g., research vs. surveillance). We recommend that programs review their country’s registration requirements well ahead of planned implementation (including timeline for registration, costs of registration, and technical requirements) to ensure there is sufficient time to complete this process before the activity is rolled out. In some countries, a waiver of registration may be granted for research or surveillance purposes
No. Given that the test has been evaluated and validated in ILB/CDC and other independent laboratories (e.g., NICD, South Africa), in-country validation of the RTRI is not necessary prior to implementation of recent infection surveillance. Validation of this test may not be feasible in every country since it requires a well-characterized bank of specimens with known HIV status (positive or negative) and recent infection status (recent or long-term), including longitudinal panels from seroconverting persons. Close review of routinely available data helps to monitor performance of the test and progress of implementation. If necessary, CDC HQ may be able to provide a verification panel and support for in-country verification of the RTRI.
Yes. A generic protocol template for HIV recent infection surveillance is available for countries to adapt to guide implementation and to use for required review and approval by the host country government, CDC, DOD, and other partner institutions. Protocols may be deemed non-research public health surveillance, depending on scope, objectives, design and procedures. Host country local institutional review board (IRB) review may be desirable or necessary. Country teams are encouraged to submit their protocols to the local IRB at least 6 months prior to planned implementation to ensure there is sufficient time to obtain necessary approvals before the activity begins.
For further questions or assistance, please contact Recency Surveillance Co-Leads:
Bharat Parekh, [email protected]
Monita Patel, [email protected]
Helen Dale, [email protected]
- Parekh B, Detorio M, Shanmugam V, Yufenyuy E, Dobbs T, Kim A, Nkengasong J. Performance evaluation of Asante Rapid Recency Assay for HIV diagnosis and detection of recent infection: potential for surveillance and prevention. Poster session presented at: IAS Conference on HIV Science; 2017 July 23-26; Paris, France. Available at: http://www.ias2017.org/Portals/1/Files/IAS2017_LO.compressed.pdf
- Agyemang E, et al. Performance of a novel rapid test for recent HIV infection among newly-diagnosed pregnant adolescent girls and young women in four high-HIV-prevalence districts — Malawi, 2017–2018. PLOS ONE. https://doi.org/10.1371/journal.pone.0262071
